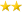hookay - haven't studied this - blood hormone test results..hard to cut/paste PDF..
Name:
LIFE EXTENSION / NATIONAL DIAGNOSTICS, INC
FINAL REPORT
6/20/2013 11:32:39 AM
*70269862*
LDH 169 IU/L 0-225 CB
Alkaline Phosphatase, S 74 IU/L 25-150 CB
Bilirubin, Total 1.1 mg/dL 0.0-1.2 CB
A/G Ratio 1.5 1.1-2.5 CB
Globulin, Total 2.6 g/dL 1.5-4.5 CB
Albumin, Serum 4.0 g/dL 3.5-5.5 CB
Protein, Total, Serum 6.6 g/dL 6.0-8.5 CB
Phosphorus, Serum 3.1 mg/dL 2.5-4.5 CB
Calcium, Serum 8.8 mg/dL 8.7-10.2 CB
**Please note reference interval change**
Carbon Dioxide, Total 20 mmol/L 20-32 CB
Chloride, Serum 106 mmol/L 97-108 CB
Potassium, Serum 4.4 mmol/L 3.5-5.2 CB
Sodium, Serum 140 mmol/L 134-144 CB
BUN/Creatinine Ratio 18 9-20 CB
eGFR If Africn Am 94 mL/min/1.73 >59 CB
eGFR If NonAfricn Am 81 mL/min/1.73 >59 CB
Creatinine, Serum 1.01 mg/dL 0.76-1.27 CB
BUN 18 mg/dL 6-24 CB
Uric Acid, Serum 6.7 mg/dL 3.7-8.6 CB
Glucose, Serum 176 High mg/dL 65-99 CB
CMP14+LP+4AC+CBC/D/Plt 164-305-1044-0
FONOROW, OWEN - ID#: 319674
Tests Result Flag Units Reference Interval Lab
OWEN FONOROW 319674USPS First-Class 74488778OWEN FONOROW 70269862OWEN FONOROW
LIFE EXTENSION / NATIONAL DIAGNOSTICS, INC
Address Account Address
01/02/1954 59 M Yes Wolff E 1346232089
CMP14+LP+4AC+CBC/D/Plt; PSA Total+% Free (Serial); FSH and LH; Testosterone,Free and Total; Pregnenolone,
Class 74488778OWEN FONOROW 70269862OWEN FONOROW
LIFE EXTENSION / NATIONAL DIAGNOSTICS, INC
Address Account Address
CMP14+LP+4AC+CBC/D/Plt; PSA Total+% Free (Serial); FSH and LH; Testosterone,Free and Total; Pregnenolone, MS; Dihydrotestosterone; Thyroxine (T4)
Tests Ordered
5990 NORTH FEDERAL HIGHWAY, FT. LAUDERDALE, FL 33308
FONOROW 319674 164-305-1044-0 6/13/2013 8:36 AM 6/13/2013 6/20/2013 6:08 AM
Last Name Lab ID Specimen Number Time Collected Date Entered Time Reported
LAB RESULTS
Date of Birth Age Sex Fasting Physician Name Physician ID
First Name Middle Initial Phone Control Number Account Number Account Phone Number
This document contains private and confidential health information protected
by state and federal law. If you have received this document in error, please
call 800-208-3444.
LIFE EXTENSION / NATIONAL DIAGNOSTICS, INC
FINAL REPORT
6/20/2013 11:32:39 AM
Monocytes(Absolute) 0.3 x10E3/uL 0.1-1.0 CB
Lymphs (Absolute) 1.4 x10E3/uL 0.7-4.5 CB
Neutrophils (Absolute) 2.6 x10E3/uL 1.8-7.8 CB
Immature Cells CB
Basos 0 % 0-3 CB
Eos 2 % 0-7 CB
Monocytes 8 % 4-13 CB
Lymphs 33 % 14-46 CB
Neutrophils 57 % 40-74 CB
Unable to perform an accurate platelet count due to aggregation of
the platelets.
Platelets TNP x10E3/uL CB
RDW 16.3 High % 12.3-15.4 CB
MCHC 36.8 High g/dL 31.5-35.7 CB
MCH 29.8 pg 26.6-33.0 CB
MCV 81 fL 79-97 CB
Hematocrit 36.1 Low % 37.5-51.0 CB
Hemoglobin 13.3 g/dL 12.6-17.7 CB
Red blood cells appear slightly agglutinated
RBC 4.46 x10E6/uL 4.14-5.80 CB
WBC 4.4 x10E3/uL 4.0-10.5 CB
Avg.Risk 5.0 4.4
2X Avg.Risk 9.6 7.1
1/2 Avg.Risk 3.4 3.3
T. Chol/HDL Ratio
Men Women
3X Avg.Risk 23.4 11.0
diabetes, severe obesity, and family history of premature
CHD.
factors affect CHD Risk such as hypertension, smoking,
.
The CHD Risk is based on the T. Chol/HDL ratio. Other
Estimated CHD Risk 0.9 times avg. 0.0-1.0 CB
T. Chol/HDL Ratio 4.7 ratio units 0.0-5.0 CB
Comment: CB
LDL Cholesterol Calc 95 mg/dL 0-99 CB
VLDL Cholesterol Cal 22 mg/dL 5-40 CB
According to ATP-III Guidelines, HDL-C >59 mg/dL is considered a
negative risk factor for CHD.
HDL Cholesterol 32 Low mg/dL >39 CB
Triglycerides 112 mg/dL 0-149 CB
Cholesterol, Total 149 mg/dL 100-199 CB
Iron, Serum 59 ug/dL 40-155 CB
ALT (SGPT) 38 IU/L 0-44 CB
AST (SGOT) 19 IU/L 0-40 CB
CMP14+LP+4AC+CBC/D/Plt 164-305-1044-0
FONOROW, OWEN - ID#: 319674
Tests Result Flag Units Reference Interval Lab
This document contains private and confidential health information protected
by state and federal law. If you have received this document in error, please
call 800-208-3444.
LIFE EXTENSION / NATIONAL DIAGNOSTICS, INC
FINAL REPORT
6/20/2013 11:32:39 AM
Reference Range:
Adults: <151
Pregnenolone, MS 16 ng/dL ES
Pregnenolone, MS 164-305-1044-0
Free Testosterone(Direct) 3.1 Low pg/mL 7.2-24.0 BN
Testosterone, Serum 124 Low ng/dL 348-1197 CB
Testosterone,Free and Total 164-305-1044-0
FSH 3.8 mIU/mL 1.5-12.4 CB
LH 0.6 Low mIU/mL 1.7-8.6 CB
FSH and LH 164-305-1044-0
% Free PSA 50-64 yr 65-75 yr
0.00-10.00% 56% 55%
10.01-15.00% 24% 35%
279:1542).
The table below lists the probability of prostate cancer for
men with non-suspicious DRE results and total PSA between
4 and 10 ng/mL, by patient age (Catalona et al, JAMA 1998,
recommendations regarding the use of
percent free PSA for any other population
of men.
Please note: Catalona et al did not make specific
15.01-20.00% 17% 23%
20.01-25.00% 10% 20%
>25.00% 5% 9%
% Free PSA 9.4 % CB
Roche ECLIA methodology.
PSA, Free 0.32 ng/mL N/A CB
decrease and remain at undetectable levels after radical
prostatectomy. The AUA defines biochemical recurrence as an initial
According to the American Urological Association, Serum PSA should
Roche ECLIA methodology.
.
interchangeably. Results cannot be interpreted as absolute evidence
of the presence or absence of malignant disease.
Values obtained with different assay methods or kits cannot be used
PSA value 0.2 ng/mL or greater followed by a subsequent confirmatory
PSA value 0.2 ng/mL or greater.
Prostate Specific Ag, Serum 3.4 ng/mL 0.0-4.0 CB
PSA Total+% Free (Serial) 164-305-1044-0
Verified by microscopic examination.
Hematology Comments: Note: CB
NRBC CB
Immature Grans (Abs) 0.0 x10E3/uL 0.0-0.1 CB
Immature Granulocytes 0 % 0-2 CB
Baso (Absolute) 0.0 x10E3/uL 0.0-0.2 CB
Eos (Absolute) 0.1 x10E3/uL 0.0-0.4 CB
CMP14+LP+4AC+CBC/D/Plt 164-305-1044-0
FONOROW, OWEN - ID#: 319674
Tests Result Flag Units Reference Interval Lab
This document contains private and confidential health information protected
by state and federal law. If you have received this document in error, please
call 800-208-3444.
LIFE EXTENSION / NATIONAL DIAGNOSTICS, INC
FINAL REPORT
6/20/2013 11:32:39 AM
Sex Horm Binding Glob, Serum 18.8 Low nmol/L 19.3-76.4 CB
Seemed to miss some tests first timeSex Horm Binding Glob, Serum 164-305-1044-0
Triiodothyronine,Free,Serum 3.6 pg/mL 2.0-4.4 CB
Triiodothyronine,Free,Serum 164-305-1044-0
Estrogens, Total 55 pg/mL 40-115 BN
Estrogens, Total 164-305-1044-0
Progesterone 0.2 ng/mL 0.2-1.4 CB
Progesterone 164-305-1044-0
IGF-BP3 4.8 mg/L 3.4-6.9 BN
IGF-BP3 164-305-1044-0
Coenzyme Q10, Total 0.99 ug/mL 0.37-2.20 BN
Coenzyme Q10, Total 164-305-1044-0
The Endocrine Society went on to further define vitamin D
insufficiency as a level between 21 and 29 ng/mL (2).
1. IOM (Institute of Medicine). 2010. Dietary reference
Vitamin D deficiency has been defined by the Institute of
Medicine and an Endocrine Society practice guideline as a
level of serum 25-OH vitamin D less than 20 ng/mL (1,2).
Evaluation, treatment, and prevention of vitamin D
deficiency: an Endocrine Society clinical practice
guideline. JCEM. 2011 Jul; 96(7):1911-30.
intakes for calcium and D. Washington DC: The
National Academies Press.
2. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al.
Vitamin D, 25-Hydroxy 49.3 ng/mL 30.0-100.0 CB
Vitamin D, 25-Hydroxy 164-305-1044-0
Reverse T3, Serum 15.0 ng/dL 9.2-24.1 BN
Reverse T3, Serum 164-305-1044-0
Insulin-Like Growth Factor I 128 ng/mL 51-194 BN
IGF-1 164-305-1044-0
Roche ECLIA methodology
Estradiol 25.3 pg/mL 7.6-42.6 CB
Estradiol 164-305-1044-0
TSH 2.390 uIU/mL 0.450-4.500 CB
TSH 164-305-1044-0
DHEA-Sulfate 69.5 ug/dL 51.7-295.0 CB
DHEA-Sulfate 164-305-1044-0
T4,Free(Direct) 1.28 ng/dL 0.82-1.77 CB
Thyroxine (T4) Free, Direct, S 164-305-1044-0
Reference Range:
Adult Male: 30 - 85
Dihydrotestosterone 13 Low ng/dL ES
Dihydrotestosterone 164-305-1044-0
FONOROW, OWEN - ID#: 319674
Tests Result Flag
Owen R. Fonorow
HeartCURE.Info CARDIO-C.COM VITC-STORE.COM
LifeWave.COM/vitamincfoundation (Partner ID 2486278)
LifeWave.COM/inteligentVitaminC (Partner ID 2533974)